A versatile platform tablet formulation technology
- The Surge Dose® formulation approach is applicable to most small molecules that are absorbed following oral administration
- Allows for the development of tablets with faster and more consistent oral absorption for every patient, every time
- Starting point for formulation development based on biopharmaceutics properties of the API
- Surge Dose® leverages any pH dependent solubility effects to maximise the rate and extent of release, independent of the gastric pH and to improve bioavailability
- Suitable for the development of NMEs, lifecycle management for marketed drugs or 505(b)(2) programs

Proprietary non-sink dissolution approach
- Rapid formulation optimisation achieved using proprietary non-sink dissolution approach correlated with rapid in vivo absorption which reduces development times and costs
- Discriminating methods demonstrate the extent to which in vivo disintegration and dissolution can contribute to bioavailability improvement and absorption variability
- Methods use limited acid comparable to in vivo gastric acid quantities which allows pH changes during dissolution, with low or no stir conditions simulating gut stasis
- Effective development tool using standard dissolution equipment to provide quantitative baseline data on existing products and experimental formulations
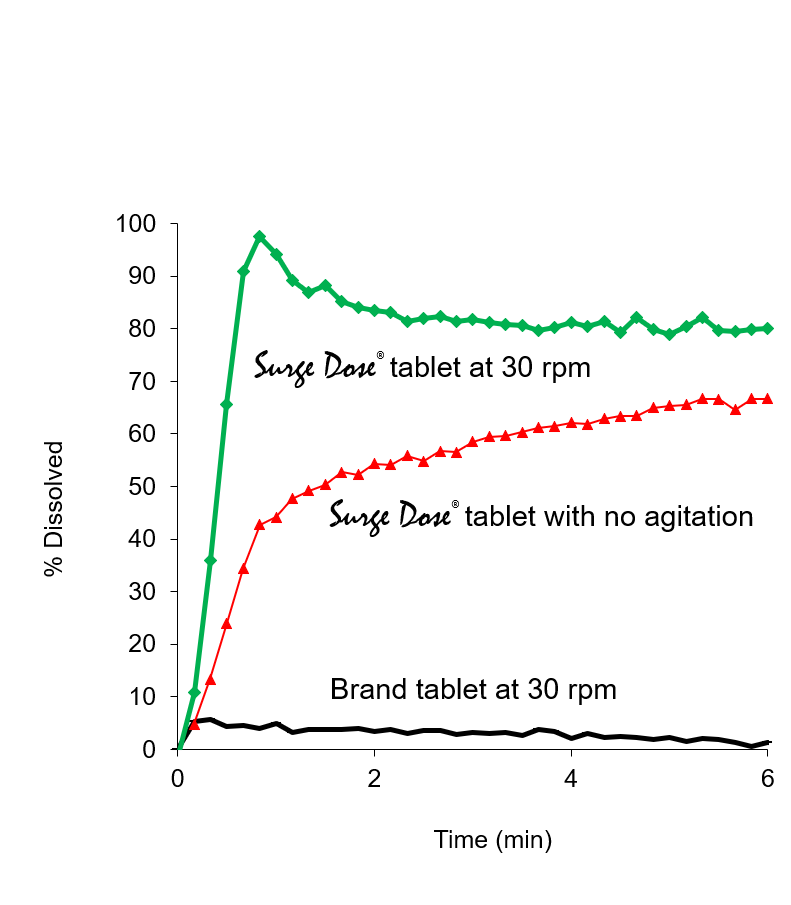
Proprietary non-sink dissolution methodology provides an effective and discriminating development tool
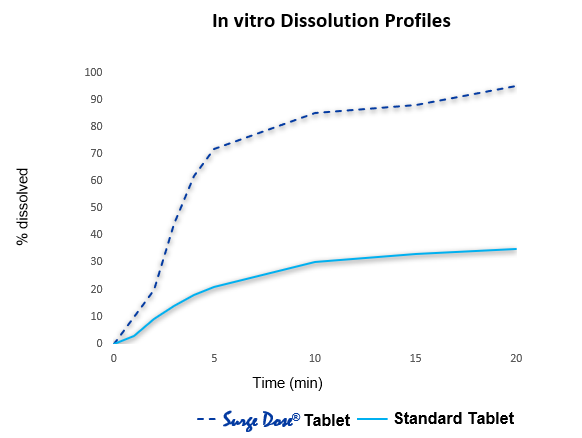
- Surge Dose® formulation specification is 50-70% rapid dissolution for all test conditions including neutral pH and no stirring
- By comparison, tablets based on conventional formulations usually show 0-10% dissolution under the same non-sink dissolution conditions
- Dissolution methods quickly identify lead formulations for stability testing and clinical evaluation, thereby significantly reducing development time, costs and risks
- Provides quantitative data to optimise formulations and identify potentially novel combinations for new IP
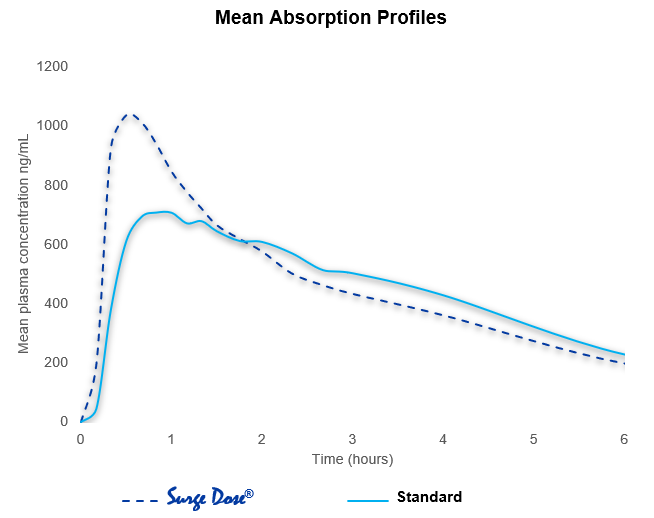
Ultra-fast activated dissolution correlates with fast, consistent absorption
Demonstrated in vivo for paracetamol used for pain and fever, and for the NSAIDs (non-steroidal anti-inflammatory drugs) lornoxicam, diclofenac, ibuprofen and naproxen used for pain, fever and inflammation.
Improves dissolution for many different drugs
- PK data for many drugs show wide absorption variability with Tmax values ranging from 30 minutes to 3 hours that are related to slow in-vivo dissolution and gastric emptying
- Non-sink dissolution methods readily demonstrate the suitability of the Surge Dose® formulation technology to improve the rate of in vitro and in vivo dissolution any drug or drug combination
- Reformulation to meet the Surge Dose® dissolution specifications will result in faster and more consistent absorption
- Consistent absorption for every dose will translate to real patient benefits and improved clinical outcomes
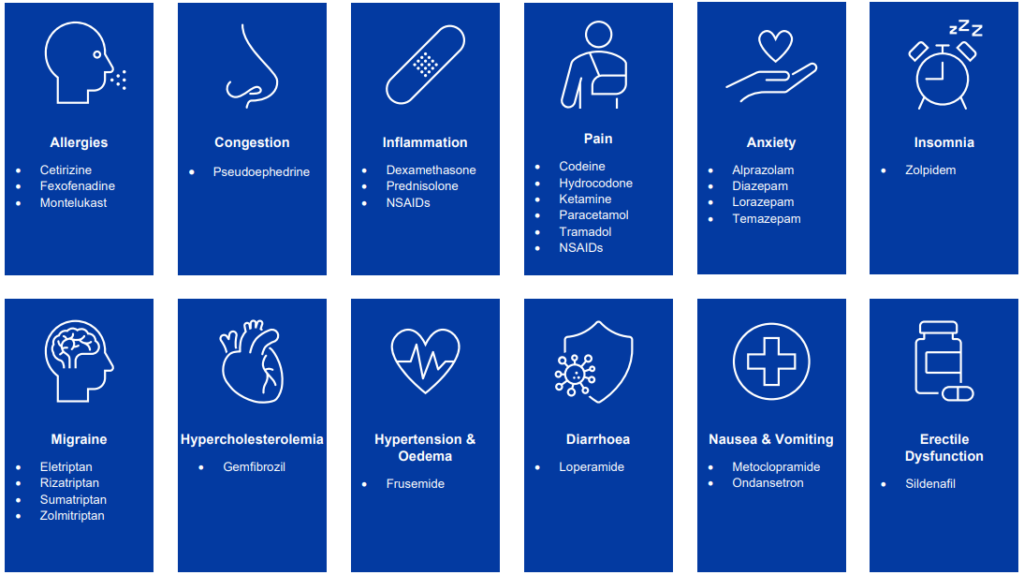
- Surge Dose® formulations of the following drugs have been shown to significantly increase dissolution rates, which will result in faster and more consistent absorption, leading to improved clinical outcomes