Sets a new standard for fast and consistent oral absorption.
The extent of absorption variability from one dose to another following oral administration of drugs is little recognised, hidden behind mean plasma absorption profiles and traditional statistical analysis of pharmacokinetic data.
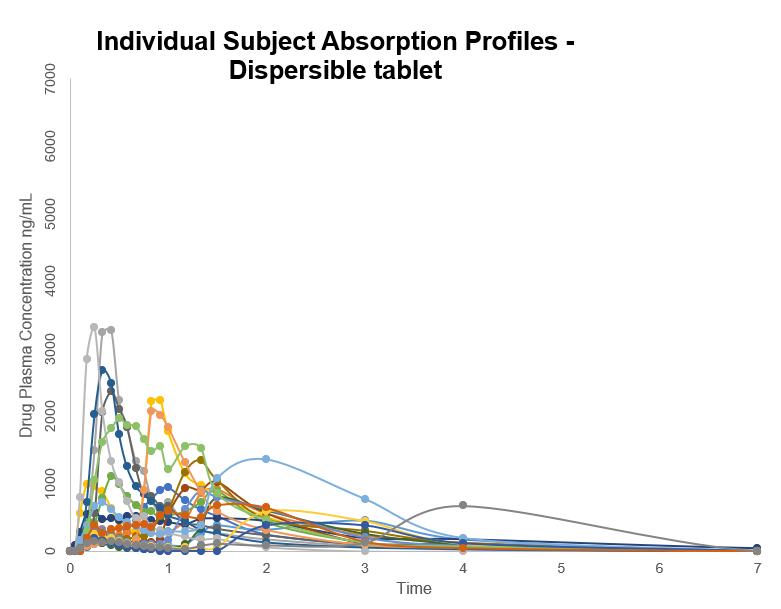
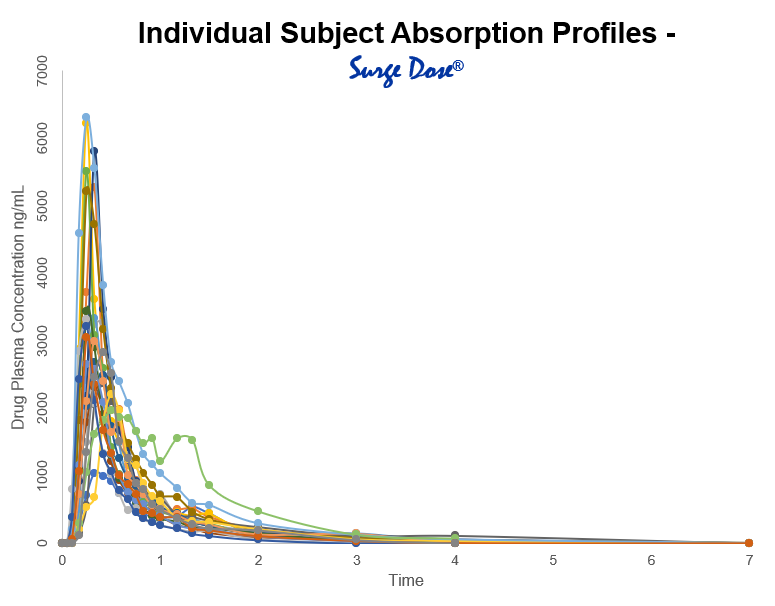
Individual subject absorption profiles show that for the same product taken by the same person, peak plasma levels of the drug can vary significantly and the time to peak can vary from 15 minutes to 2 hours or more. When slow or delayed absorption occurs, peak plasma levels are low and may not even reach minimum therapeutic levels with associated lack of efficacy which is particularly important for drugs used in acute indications such as pain.
Through its research program, Imaginot recognised that much of the absorption variability seen with solid dosage forms can be attributed to absorption rate-limiting drug release and dissolution in the stomach. This is further influenced by gastric pH, and the gastric emptying cycle that transfers drug from the stomach to the small intestine whence absorption occurs. This led to the development of the Surge Dose® tablet formulation technology which reduces the variability and improves in vivo performance by working with the gut physiology to achieve fast and consistent absorption for every patient and every dose.
achieves fast and consistent absorption from swallow tablets
Although injections are an alternative to avoid the variability in plasma levels following oral absorption these are not practical and have very low patient acceptability despite offering improved clinical outcomes.
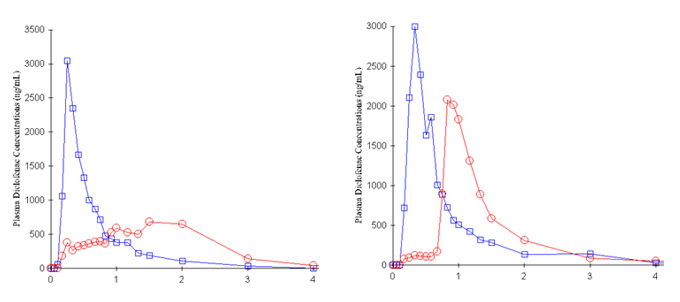
Use of dispersible tablets, mixed with water before swallowing and ODTs which eliminate in vivo disintegration and dissolution as absorption-rate limiting factors still show highly variable absorption. A direct comparison of a swallow Surge Dose® tablet with the dispersible brand leader tablet showed many instances of delayed absorption and multiple peaks for the dispersible product compared faster and more consistent absorption for the Surge Dose® tablet. Individual subject absorption profiles compare the dispersed tablet (red) with the Surge Dose® tablet (blue). The delayed peaks with the dispersed tablet result from retention of the drug in the stomach until gastric emptying transfers it into the small intestine. Drug from the Surge Dose® tablet rapidly dissolves in the co-administered water and empties into the small intestine with the water independent of gastric emptying.
Individual subject profiles of Surge Dose® (blue) compared with the standard tablet (red) showing the impact of slow in vivo disintegration and dissolution, and gastric emptying effects.
Achieves Tmax in 30 minutes for more patients
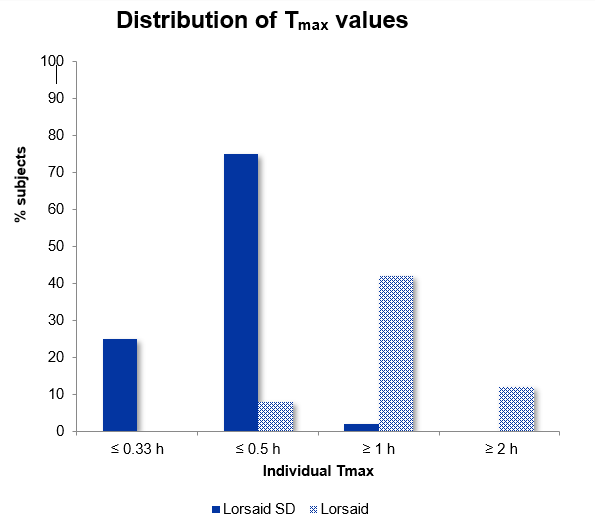
In a fasted study, comparing a Surge Dose® tablet with the market leader as the reference product:
- Surge Dose® reduced the mean time to peak plasma concentration (Tmax) from 63.6 minutes for the conventional tablet to 30.6 minutes
- The range of Tmax values with Surge Dose® was 15 – 60 minutes compared with 15 – 220 minutes
- 98% of subjects taking the Surge Dose® tablets achieved Tmax within 30 minutes and 100% within 60 minutes
- Only 2 subjects (8%) taking the market leader tablet achieved Tmax within 30 minutes, with 9 subjects (38%) showing Tmax values longer than an hour
These results should be considered in the context of patient expectations. In acute conditions such as pain, patients generally expect to experience some level of pain relief within 30 minutes. Delayed absorption will have a negative impact on their assessment of product efficacy, leading to the conclusion that a product has not worked if they do not experience some pain relief in the first half an hour.
Surge Dose® tablets overcome this problem, so that every patient will experience timely absorption and pain relief for every dose.
achieves higher Cmax for more patients
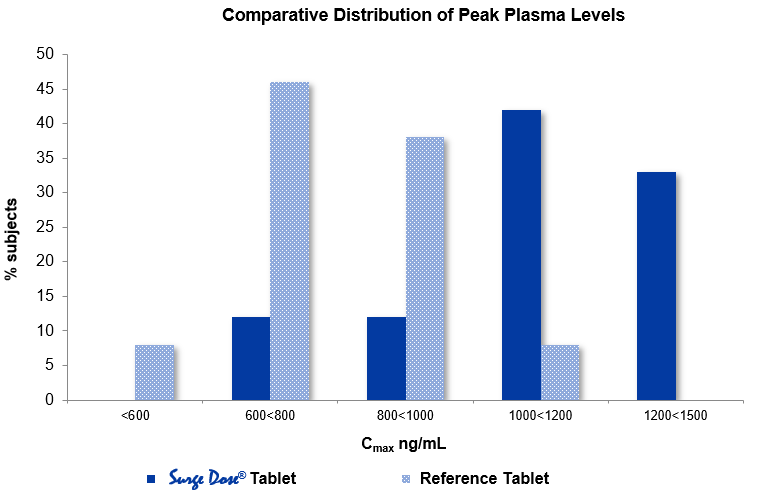
In the same study:
- Surge Dose® increased the mean peak plasma concentration (Cmax) from 788 ng/mL (range 483 – 1.087 ng/mL) for the market leader to 1,098 ng/mL (range 676 – 1,466 ng/mL).
- 100% of subjects taking the Surge Dose® tablets achieved Cmax values exceeding 600 ng/mL with 33% exceeding 1,200 ng/mL.
- While 92% of subjects taking the market leader achieved Cmax values exceeding 600 ng/mL, none exceeded 1,200 ng/mL.
Surge Dose® tablets reach minimum therapeutic plasma levels quicker and more consistently, and high concentrations drive distribution to the effect compartment.
More consistent absorption with higher Cmax values creates the opportunity to evaluate the potential for dose reduction with Surge Dose® tablets to reduce side effects without compromising efficacy.
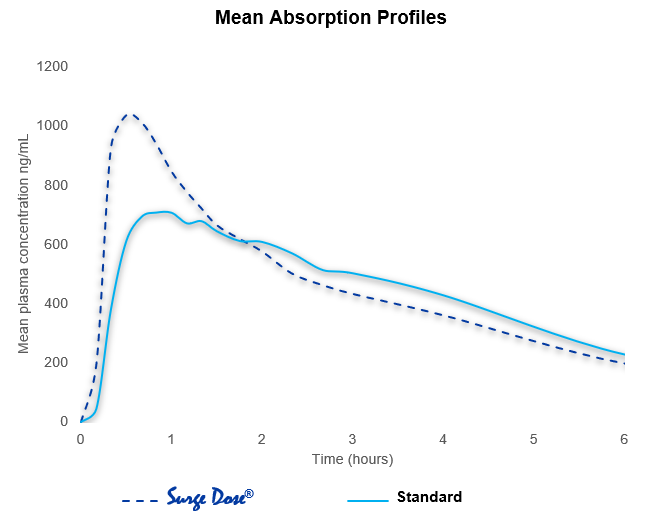
Provides fast and consistent absorption compared with conventional tablets reducing dose to dose variability
With Surge Dose® tablets, there are more subjects achieving faster, improved absorption and fewer showing multiple peaks with lower peak plasma levels compared with conventional formulations.