
A strategic formulation platform
A patented tablet technology providing faster and more consistent oral absorption for every patient, every time.
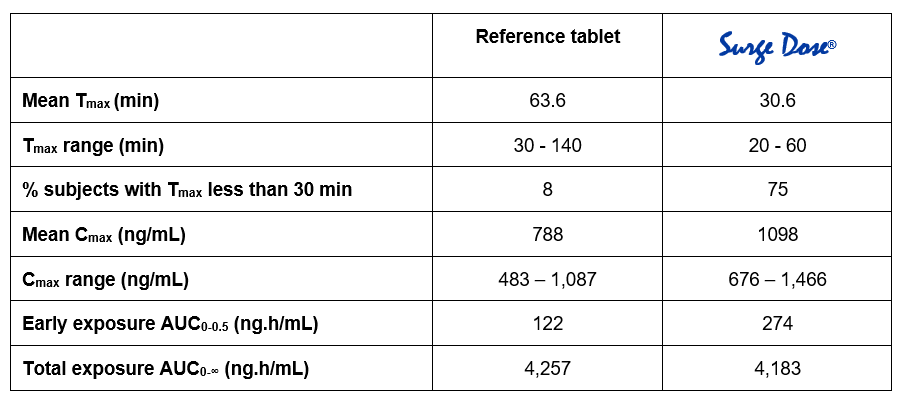
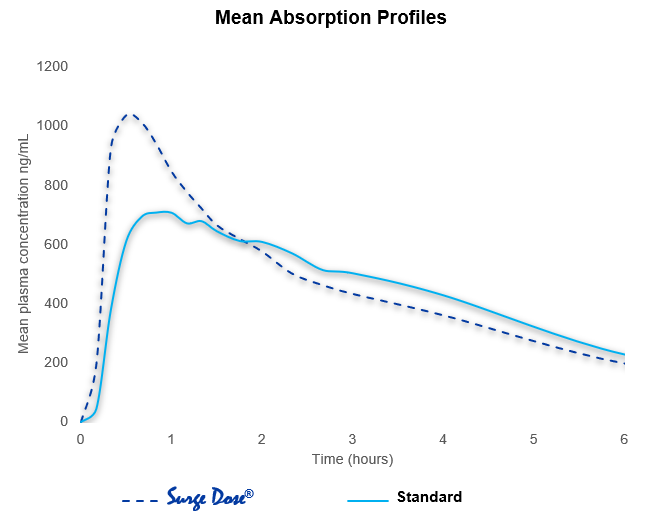
Reproducible Cmax and earlier Tmax linked directly to proactive technology interaction with gut physiology, reducing the effects of gastric emptying on absorption.
Offers lower intra- and inter-patient absorption variability with the potential for improved therapeutic outcomes.
Improves bioavailability by leveraging any pH dependent solubility effects to maximise the rate and extent of release, independent of the gastric pH.
Rapid formulation optimisation using proprietary non-sink dissolution approach correlated with in vivo absorption.
Strategy identifies lead formulations for stability testing and clinical evaluation thereby significantly reducing development time, costs and risks.
Technology uses readily available commonly used GRAS excipients with conventional manufacturing processes and equipment. Tablets can be film coated and blister packed in suitable laminates.
Superior performance compared to other ‘fast’ technologies such as ODTs, liquid filled capsules, dispersible tablets and solutions.
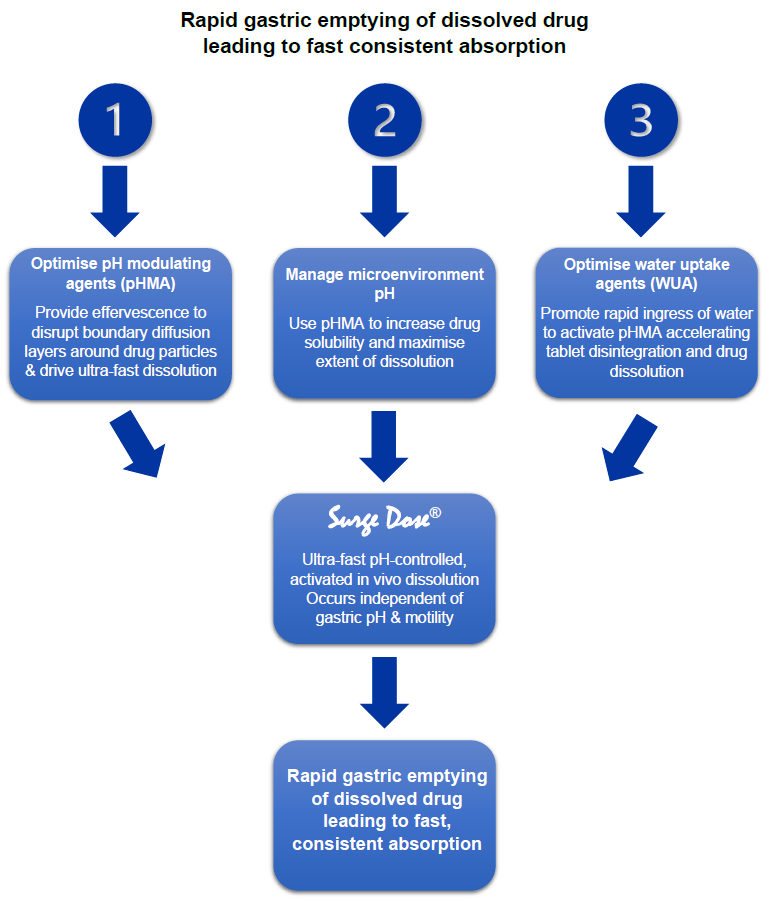
Customised levels of pH modulating agents (pHMAs) and water uptake agents (WUAs) provide ultra-fast, pH-controlled active dissolution
Levels of pHMAs such as bicarbonate with an organic acid combined with typical pharmaceutical WUAs are optimised for each drug or drug combination.
pHMAs produce effervescence that will facilitate tablet disintegration and maximise the rate of drug dissolution independent of gastric pH. They control the pH in the microenvironment of the drug particles to leverage any pH dependent solubility effects.
WUAs facilitate ingress of water into the tablet activating effervescence and bringing water into contact with drug particles to accelerate dissolution.
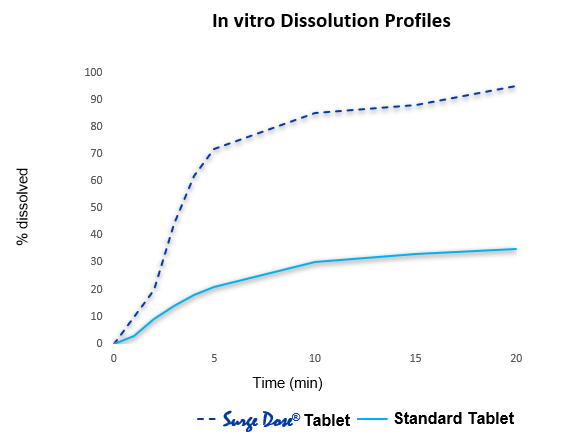
Surge Dose® formulations achieve ultrafast activated dissolution independent of pH or stirring conditions, such that drug dissolution occurs in the co-administered water
Formulation optimisation involves the use of a proprietary non-sink dissolution methodology which is an effective development tool to identify the best lead candidates for clinical evaluation.
Works with gut physiology
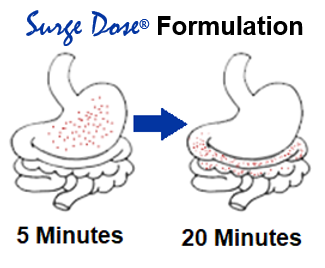
Surge Dose® tablets are swallowed whole with a glass of water. The customised blend of excipients, specific to each drug or drug combination, ensures rapid tablet disintegration and dissolution of the drug in the co-administered water. The customised excipients create the right pH to maximise drug solubility in the patient’s stomach independent of the gastric pH.
As 50% of a liquid will leave the stomach in less than 10 minutes, dissolved drug from the Surge Dose® tablet rapidly reaches the small intestine, where high drug concentrations drive drug absorption and distribution resulting in fast onset of action and improved efficacy. Absorption profiles are more consistent with the elimination of multiple peaks and slow absorption profiles caused by gastric emptying dependency.
Imaginot has demonstrated the effectiveness of its Surge Dose® technology with several marketed drugs producing consistent and fast absorption.
Ultra-fast active in vitro dissolution correlates with fast, consistent absorption in vivo
Formulations achieving Surge Dose® ultra-fast dissolution specifications under non-sink in-vitro conditions are associated with fast and consistent absorption in vivo.
This reduced PK variability maximizes therapeutic potential and levels the playing field for all patients, providing consistent therapeutic outcomes with every dose.